Michael Addition Polymers
In the recent past, a large number of novel polymers have been synthesized via step-growth Michael addition. This type of polymerization reaction is
highly efficient and very versatile and allows for the synthesis of polymers with a wide variety of properties ranging from low Tg segmented elastomers
to high Tg engineering thermoplastics. Furthermore, many different chain architectures
can be realized including block copolymers, dendrimers, hyperbranched polymers, graft copolymers and network
polymers (thermosets). The method can also be employed to modify natural
or synthetic polymers.
Due to the large number of Michael acceptors and donors available, a
large number of unique polymers can be synthesized via Michael addition. Many of these novel polymers have found uses in diverse areas such as drug delivery systems, coatings,
inks, adhesives and composites. Some major types of Michael addition polymers are shown in the table below and are briefly discussed here.
| Polymer Class | General Structure |
| Poly(amino ester)s | 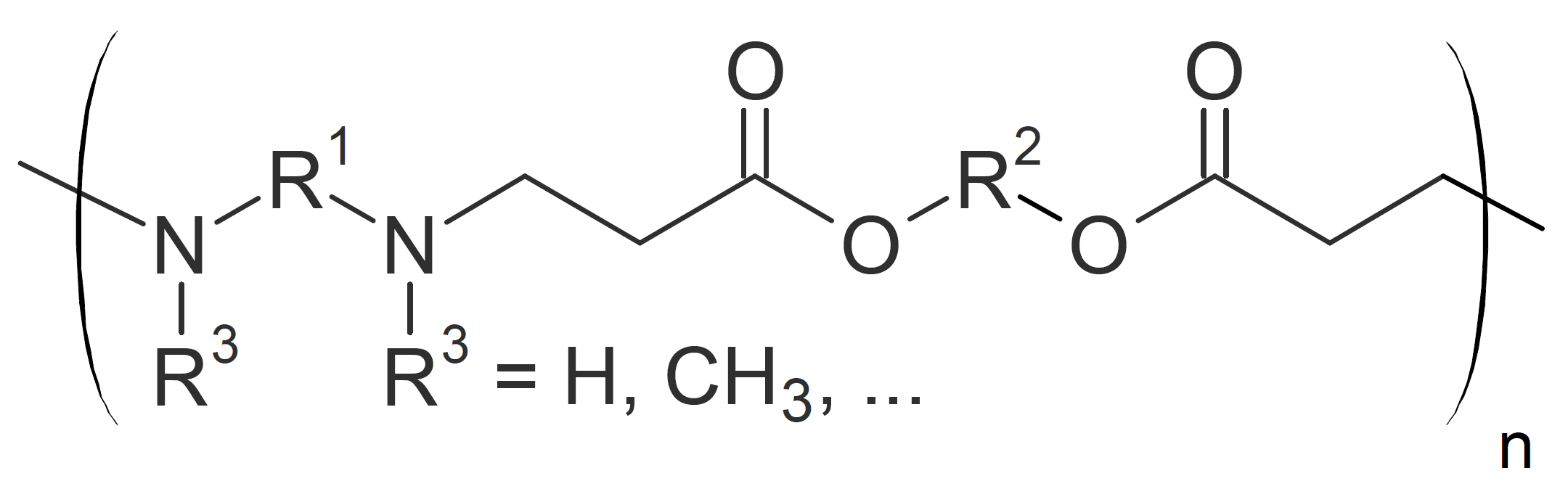 |
| Poly(amido amine)s (primary) | 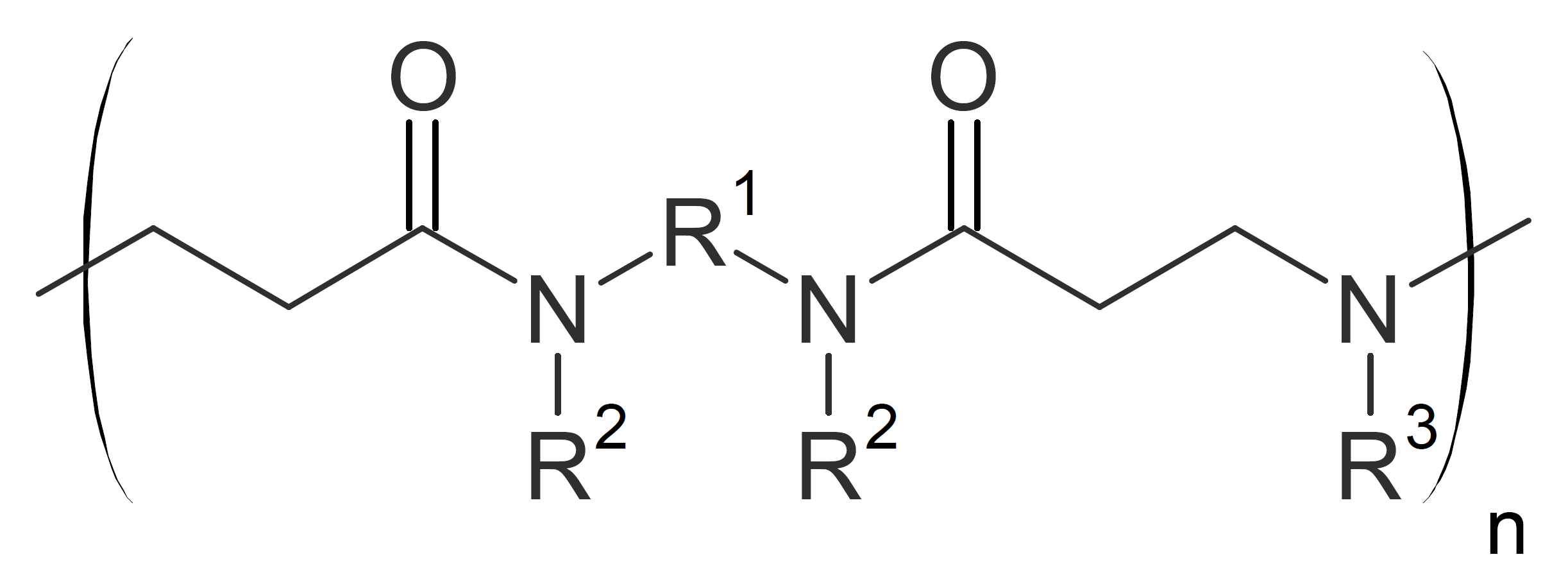 |
| Poly(amido amine)s (secondary) | 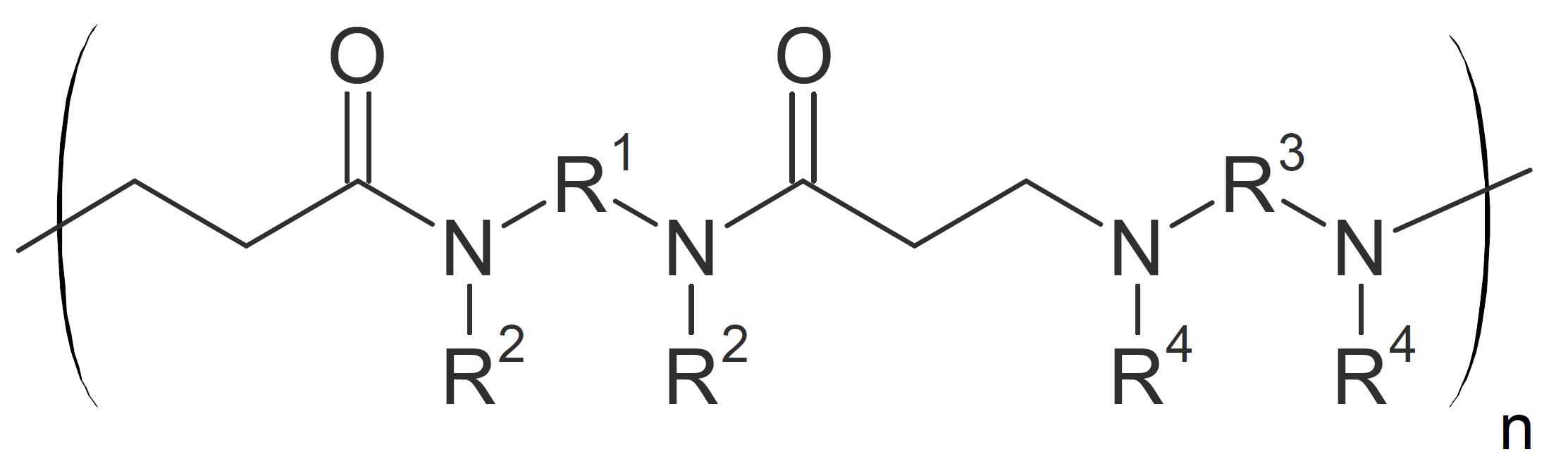 |
| Poly(ester sulfide)s | 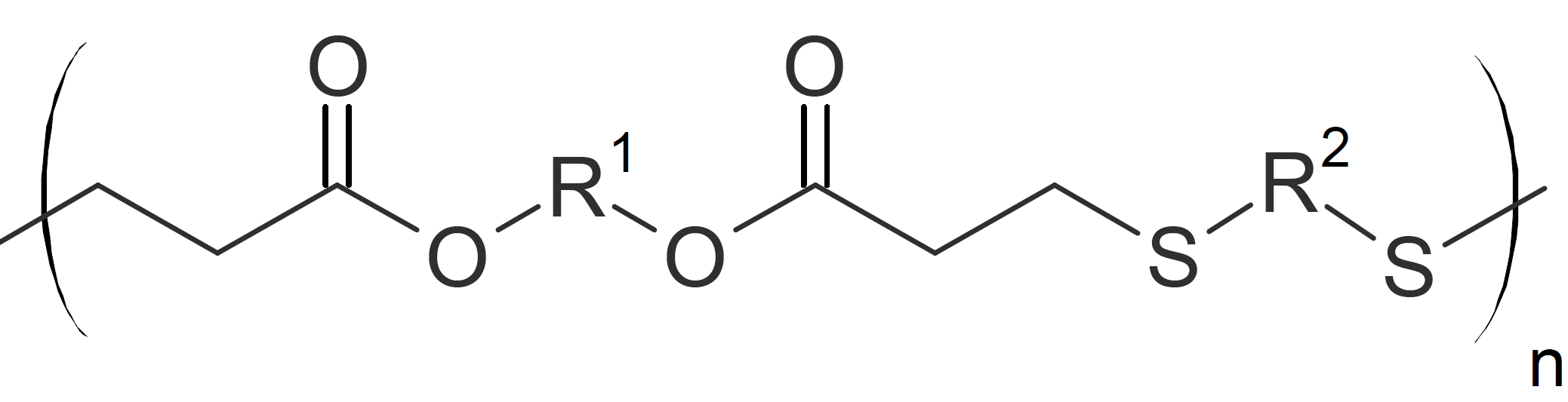 |
| Poly(ester amide)s | 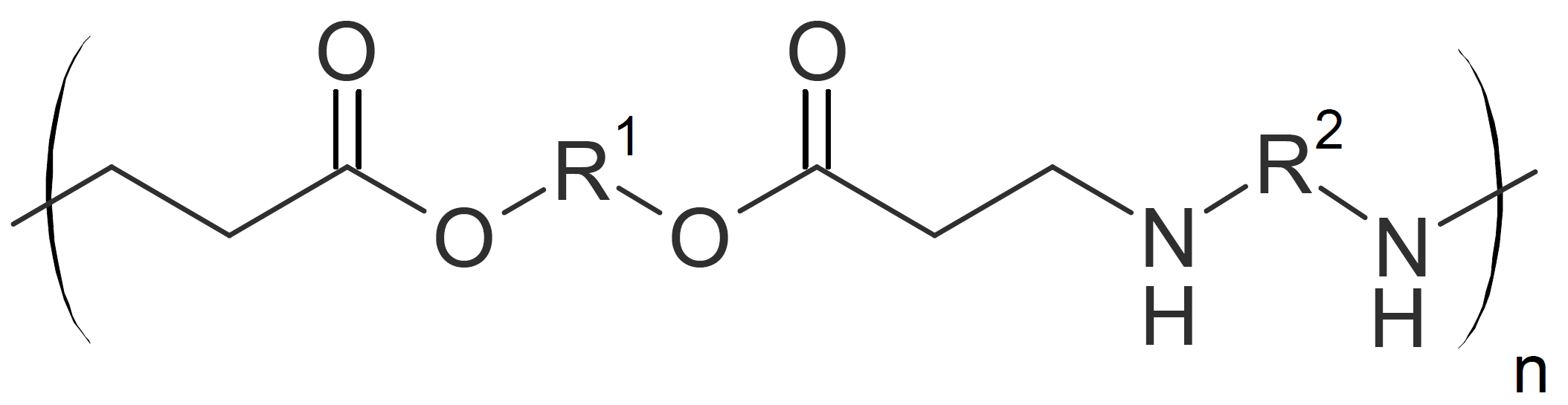 |
| Poly(imido sulfide)s | 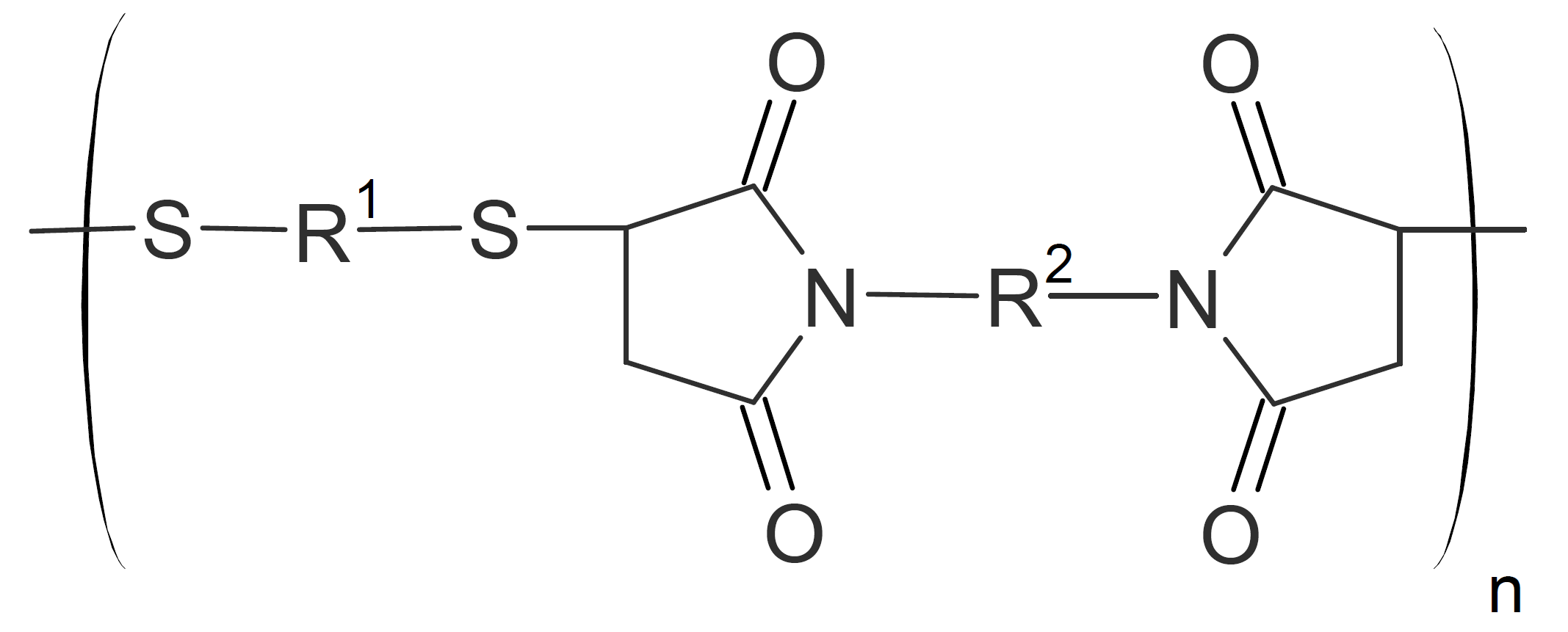 |
| Poly(aspartamide)s | 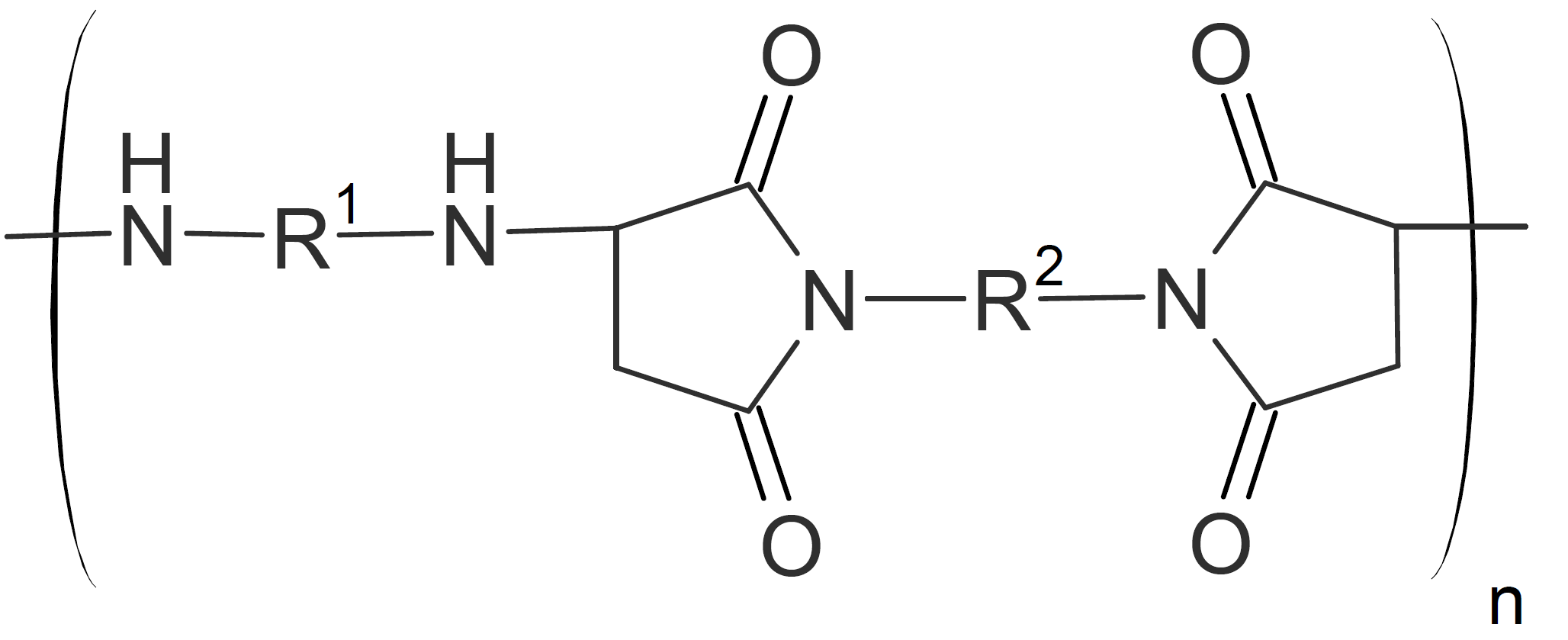 |
| Poly(aminoquinone)s Poly(thiophenylene)s |
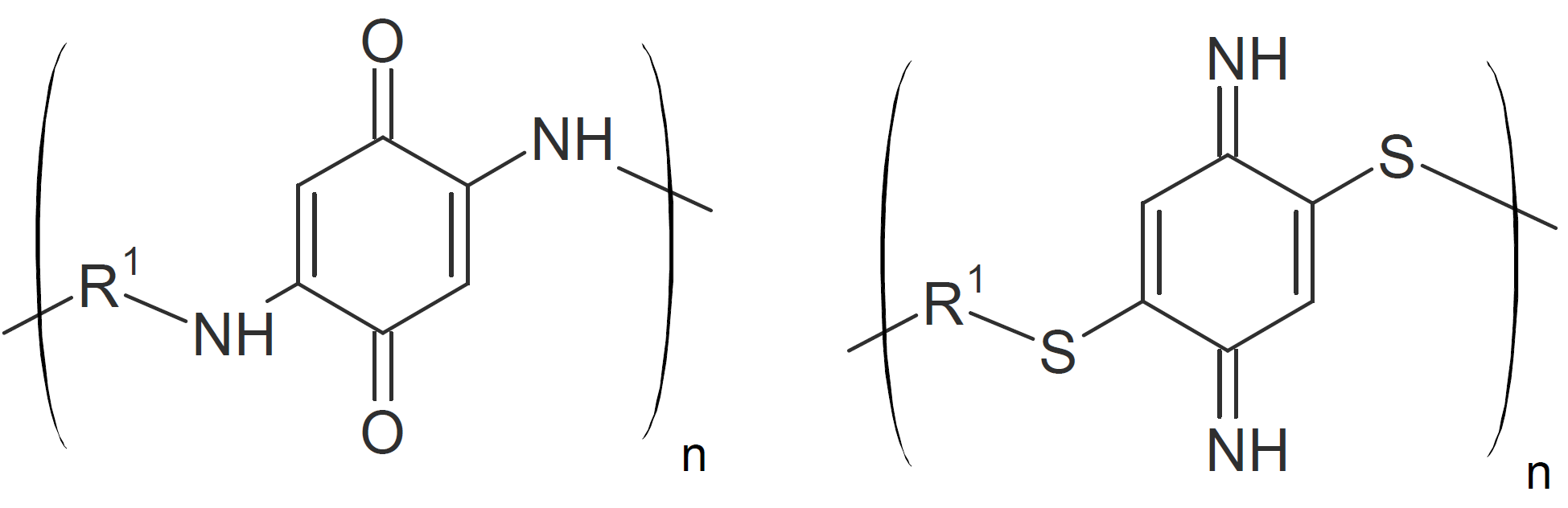 |
Polyamidoamines
Linear polyamidoamines can be synthesized by addition of secondary amines to bisacrylamides at ambient or slightly elevated temperatures. The reaction proceeds rapidly at room temperature without catalyst when aliphatic amines in protic solvents such as alcohols or water are chosen:
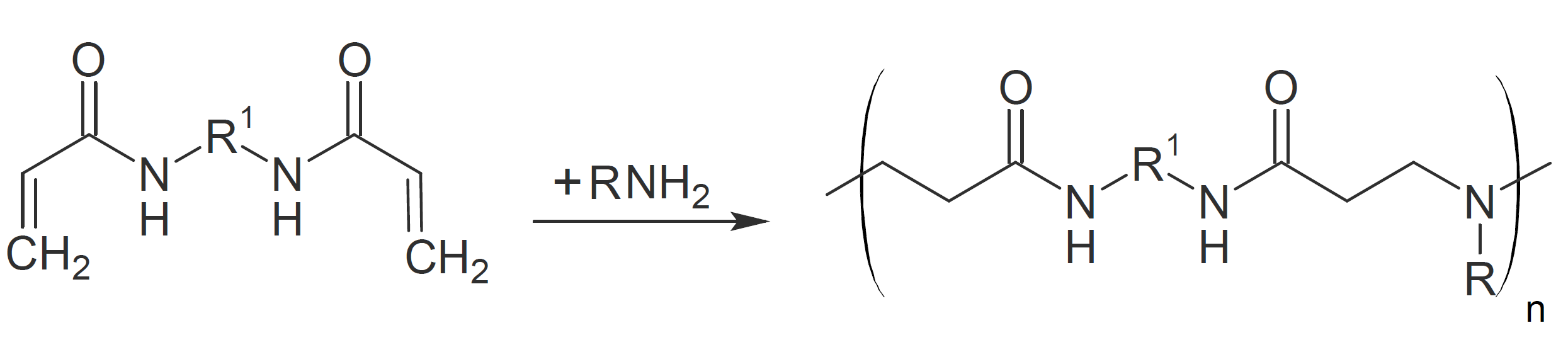
The rate of reaction typically increases with the basicity of the secondary amine and decreases with steric hindrance. Unfortunately, this type of
Michael addition typically does not yield high molecular weight polymers and the majority of aliphatic polyamidoamines synthesized by this method have relatively low decomposition temperatures
typically in the range of 200C.1 An exception are poly(amido phosphines) which decompose at much higher temperature near 360°C.1 Another drawback of these polymers is their strong
tendency toward amide hydrolysis.
The method has also been employed to produce
(hyper-)branched polymers. Due to the low probability of side reactions and mild reaction conditions, it is well suited for the
synthesis of dendrimers (PAMAM).2
Many poly(amidoamine)s are water soluble due to the high polarity of the amido groups which are partially protonated in water. Many are also susceptible to amide hydrolysis and produce only low toxic amino acid degradation products. These attributes make them interesting candidates for biological applications such as drug delivery systems.
Polyimidosulfides
Polyimido sulfides (PIS) can be synthesized by Michael addition of dithiols or hydrogen sulfide to the double bond of bismaleimides. Both aromatic and aliphatic monomers have been employed. Most PIS have moderately high decomposition temperatures in the range of 275°C to 380°C1,3 and high molecular weights (MW). The glass transition temperature can vary considerably depending on the types of monomers (aromatic vs. aliphatic) and the MW. For example, for (semi-)aromatic PIS, Tgs as high as 380°C have been reported.1 The most likely decomposition mechanism is retro-Michael addition.
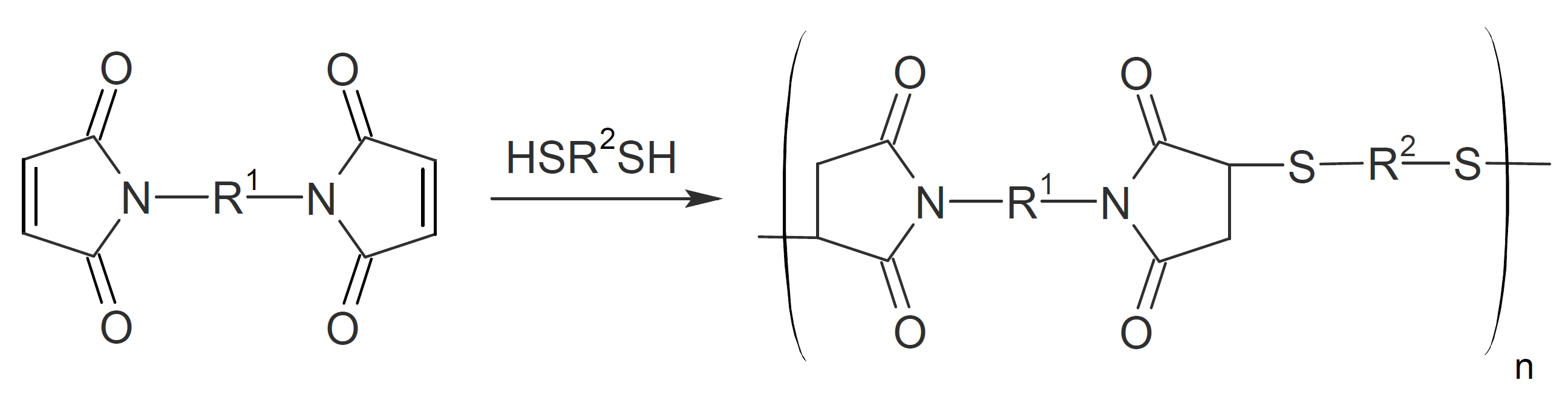
Elastomeric poly(imido sulfide)s have also been synthesized, consisting of hard and soft segments.4 Suitable soft blocks can be thiol terminated polysulfides (Thiokols) which can be Michael added to aromatic bismaleimides. This type of thermoplastic elastomer has relatively high tensile strength of up to 1500 psi (10 MPa) and ultimate elongation of 800%.4 However, these elastomers have poor thermal stability due to the low decomposition temperature of polysulfides.
PolyesterSulfides and PolyesterAmides
Poly(ester sulfide)s and poly(ester amide)s can be produced by step growth polymerization of diacrylates with dithiols or diamines, respectively.
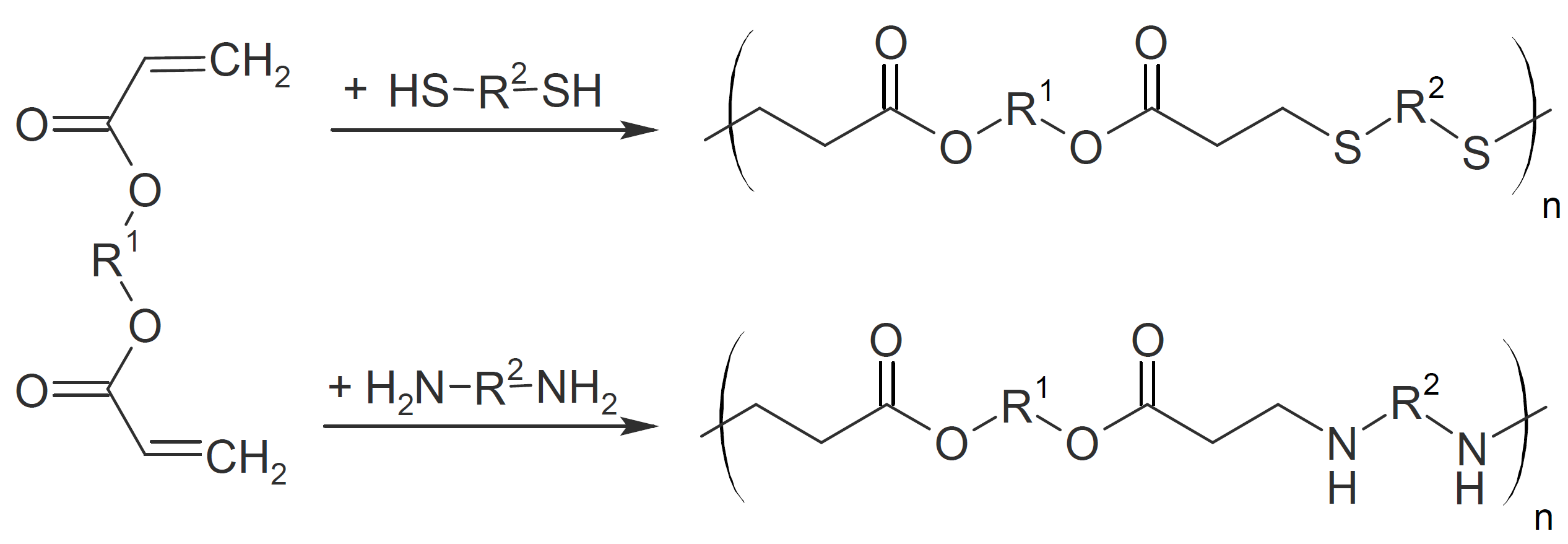
Many of these polymers have an elastomeric
character due to their very low glass transition temperature (Tg). For example, Michael addition of PEG dithiol to butandiol diacrylate in the presence of a triethylamine catalyst yields elastomers with a Tg between -50 to -60°C.5
Besides linear thermoplastics, hyperbranched poly(amine ester)s have also been synthesized. For example, dendrimers have been prepared by the reaction of diacrylates with tris(2-maleimidoethyl)amine (TMEA)6
or by the reaction of pentaerythritol tetraacrylate (PETEA) with diethanolamine (DEA) with a subsequent condensation reaction.7
These dendrimers have a well-defined structure and very low polydispersity and their preparation by Michael addition is highly efficient. Many other dendrimers have been synthesized and investigated;
these polymers have gained a lot attention by researchers due to their remarkable properties.
Polyaspartamides
Polyaspartamides are produced by condensation of bismaleimides and diamines.8 Those with fully aromatic structure have thermophysical properties very similar to polyimides including high glass transition temperature (> 200°C), and high decomposition temperature both in air and inert atmosphere (>400°C).9,10
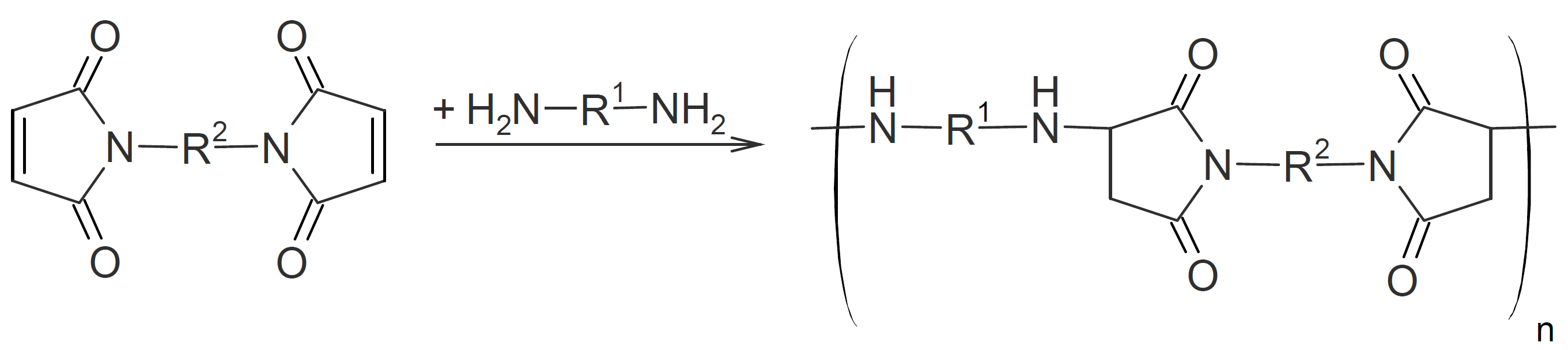
Like polyimides, fully aromatic polyaspartamides possess excellent mechanical properties. For example, tensile moduli of almost 100 MPa and flexural moduli reaching 3 GPa have been reported.1 Unlike many other high performance polymers, polyaspartamides can be produced at relatively mild conditions. For example Crivello (1973) was able to synthesize them at 110°C in cresol with catalytic amounts of acetic acid.8
Michael addition reactions have also been combined with other addition and condensation reactions. For example poly(amide aspartamide)s have been synthesized by combining Michael addition with amidation of amines and carboxylic acid. If the maleimide monomer contains both a carboxylic acid functionality and a double bond, each Michael addition step is followed by an amidation step. For example, Liu et al. prepared these polymers through the reaction of 4,4-diaminodiphenylmethane with 4-maleimidobenzoic acid or 5-maleimidoisophthalic acid, respectively.11 In a first step, the nucleophilic amine groups are added to the maleimide groups. Then amidation at the carboxylic acid is carried out in the presence of a a suitable catalyst:
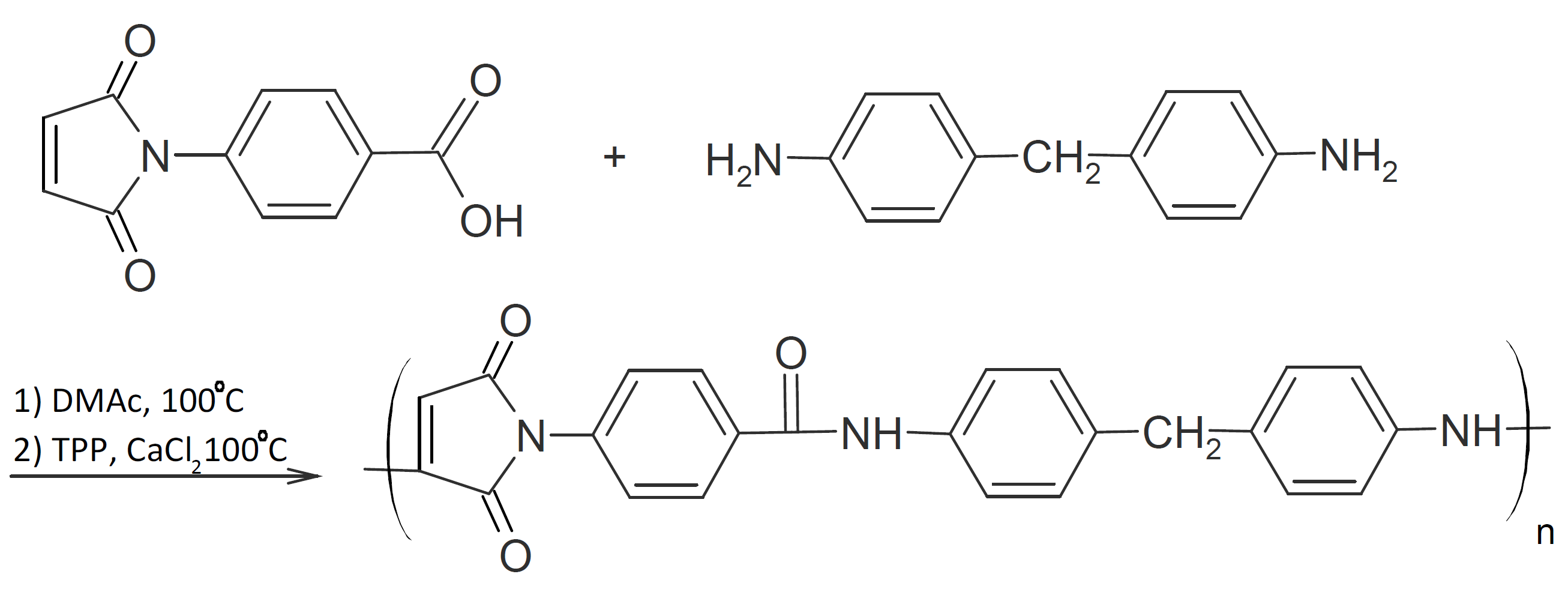
These polymers exhibit high glass transition temperatures (> 220°C) and thermal stability (> 360°C). They also possess good solubility in aprotic polar solvents such as tetrahydrofuran and acetone.1,11
Polyamino quinons
Poly(amino quinone)s can be synthesized from primary diamines and quinones through Michael addition on both double bonds of the quinone.1,12,13 Unlike most other Michael addition reactions, the double bonds of the quinone are reformed in the presence of strong oxidizing agents:1,12,14
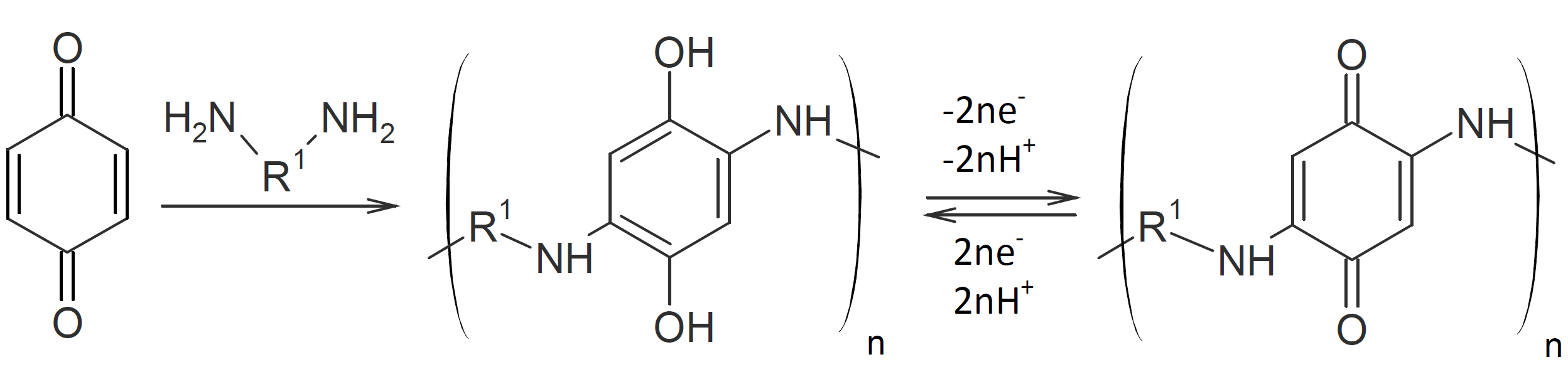
The method has also been applied to the synthesis of novel amine-quinone polyimides. For example, Han et al. (2013) produced these polymers via a three step reaction: In a first step, 4.4- methylenedianiline is reacted with 4,4-benzoquinone. The resulting intermediate is then condensed with a dianhydride such as 4,4'-benzophenone tetracarboxylic dianhydride to produce a polyamide acid which is converted in a third step to a polyimide by thermal imidization:15
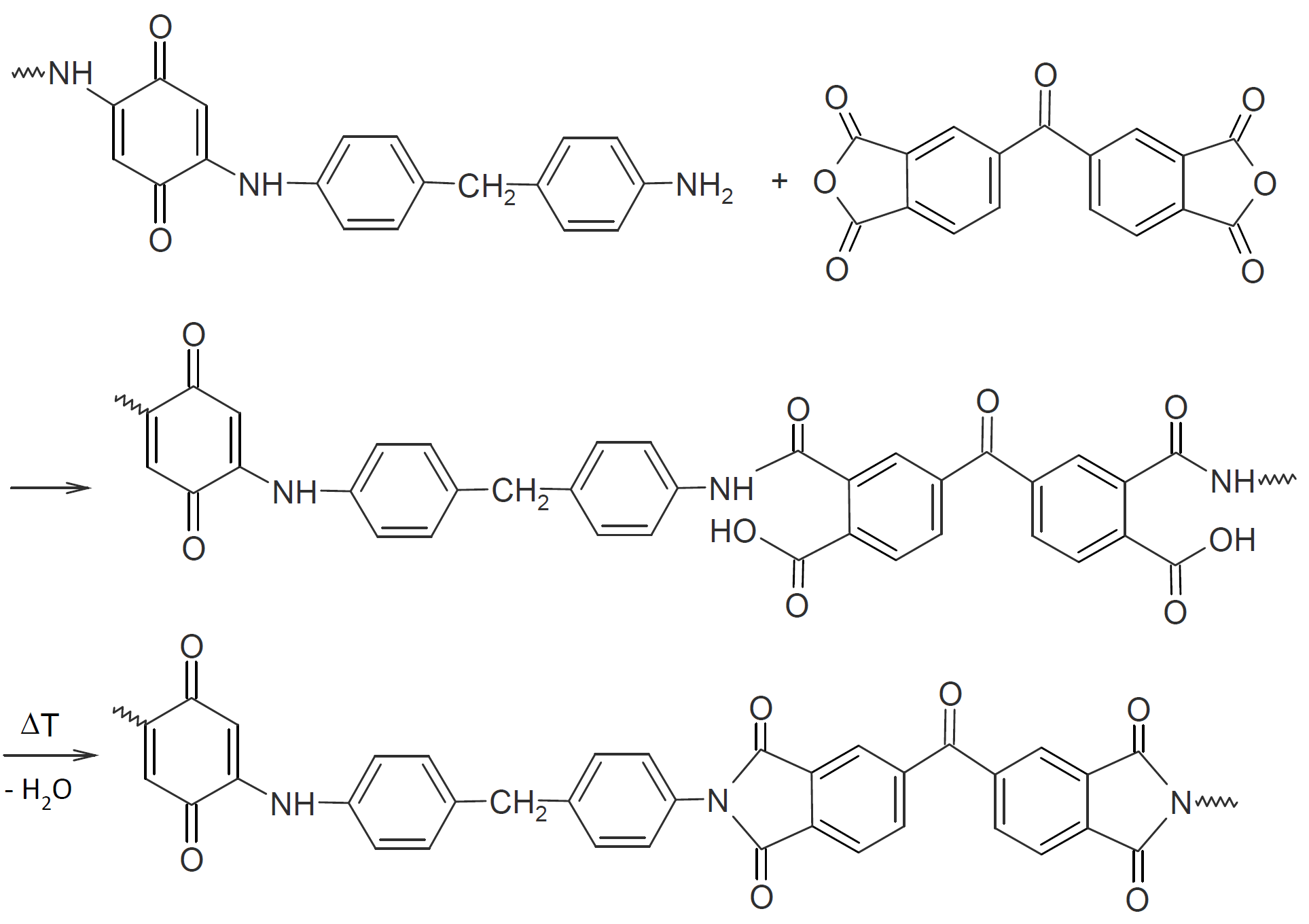
This amine-quinone polyimide (AQPI-2) has a thermal decomposition temperature of 540°C and a glass transition of 292°C.15 These values are comparable to those of typical polyimides.
Polythiophenylenes
Polythiophenylenes have been synthesized by the reaction of aromatic dithiols with bis(1,4-phenylenediimine)s:
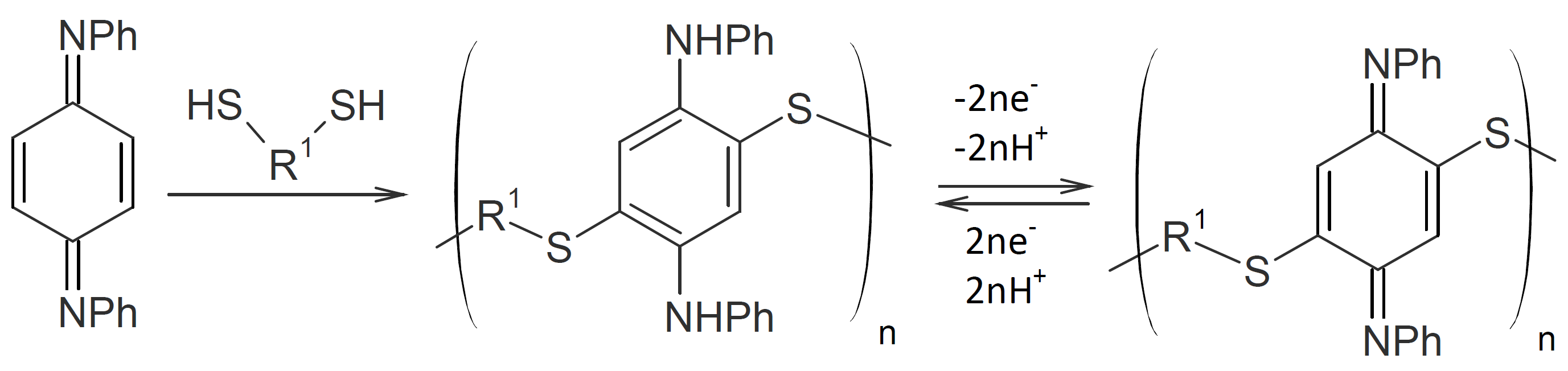
The reaction proceeds similar to quinones through proton transfer to the imine nitrogen resulting in a polythiophenylene with pendant secondary amino groups which can be converted back to imine groups by strong oxidizing agents.16
References & Notes
B.D. Mather, K. Viswanathan, K.M. Miller, T.E. Long, Prog. Polym. Sci. 31, 487-531 (2006)
D.A. Tomalia, H. Baker, J.R. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, & P. Smith, Polym. J. Vol. 17, No. 1, 117-132 (1985)
C. Gaina and V. Gaina, Des. Monomers Polym., Vol. 8, No. 2, pp. 145-158 (2005)
J.V. Crivello, P.C. Juliano, J. Polym. Sci. Polym. Chem., Vol. 13 (8), 1819-42 (1975)
S. Tomasi, R. Bizzarri, R. Solaro, E. Chiellini, J. Bioact. Compat. Polym., 17(1): 3-21 (2002)
M. Sun, C. Yin, Y. Gu, Y. Li and Z. Xin, Des. Monomers Polym., Vol. 20, No. 1, 458-467 (2017)
W. Han, B. Lin, H. Yang and X. Zhang, Des. Monomers Polym., Vol. 16, No. 1, 67-71 (2013)
J.V. Crivello, J. Polym. Sci.: Polym. Chem. Ed., Vol. 11, Issue 6, 1185-1200 (1973)
R. Hariharan, N. Amutha, S. Bhuvana and M. Sarojadevi, J. Macromol. Sci., Part A, Vol. 41 (3), 317-328 (2004)
D.P. Fasce and R.J.J. Williams, Polym. Bull., Vol. 34, 515-522 (1995)
C.S. Wu, S.H. Tsai and Y.L. Liu, J. Polym. Sci.: Part A Polym. Chem. Vol. 43 (9), 1923 - 1929 (2005)
S. Muralidharan, S. Ravichandran, S. Pitchumani, K.L.N. Phani, J. Mat. Sci. Lett. 18, 1200 - 1301 (2000)
R.F. Colletti M.J. Stewart, A.E. Taylor, N.J. MacNeill, L.J. Mathias, Polym. Chem., Vol. 29 (11), 1633 - 1638 (1991)
E. Vaccaro, D.A. Scola, Chemtech, 29, 15-23 (1999)
M. Han, H. Bie and D.E. Nikles, G.W. Warren, J. Polym. Sci.: Part A Polym. Chem. 38(16), 2893 -2899 (2000)
K. Yamamoto, M. Higuchi, H. Takai, and T. Nishiumi, Org. Lett., 3, 1, 131-134 (2001)
November 1, 2020